
However, these reactions are moderately endothermic ( E 0.59 eV, 0.52 eV, respectively). Alternatively, upon adsorption, NO can be hydrogenated to HNO or NOH. Ru is bonded to twelve equivalent Ru atoms to form a mixture of edge. NO is strongly adsorbed onto Ru (0001) (binding energy is 2.72 eV) and its decomposition into N and O atoms is energetically favorable ( E 1.44 eV). Thus, Al 2 O 3 would have a shorter interionic distance than Al 2 Se 3, and Al 2 O 3 would. Ru is Magnesium structured and crystallizes in the hexagonal P6/mmc space group.
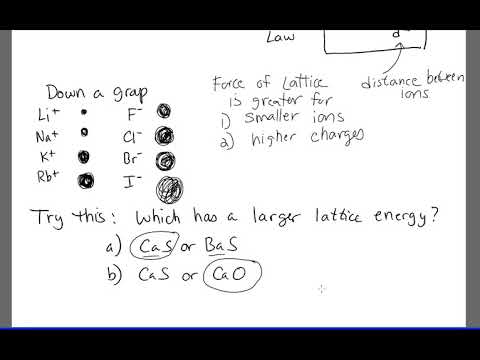
In this formula, U depicts the lattice energy of the ionic compound. The simplified form of Coulomb’s law is used to calculate the lattice energy of ionic compounds. Increasing global energy demand and ecological issues have forced us to develop green and renewable energy conversion and storage technologies, such as water electrolysis, fuel cells, and metal-air batteries. The O 2 ion is smaller than the Se 2 ion. It is not easy to compute the exact values of the lattice energy of any compound. In these two ionic compounds, the charges Z + and Z are the same, so the difference in lattice energy will mainly depend upon R o. We begin with the elements in their most common states, Cs( s) and F 2( g). Which has the larger lattice energy, Al 2 O 3 or Al 2 Se 3 Solution. Lattice energy is also known as lattice enthalpy and can be stated in two ways.

\): The Born-Haber cycle shows the relative energies of each step involved in the formation of an ionic solid from the necessary elements in their reference states.


 0 kommentar(er)
0 kommentar(er)
